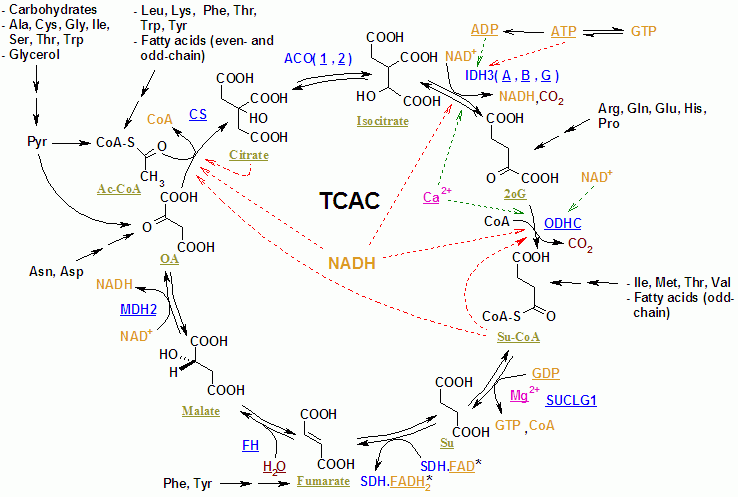
The citric acid cycle (also known as the tricarboxylic acid cycle, TCA cycle, and as the Krebs cycle) is a series of chemical reactions of central importance in all living cells that utilize oxygen to generate useful energy by cellular respiration. Essentially, the cycle involves converting the potential energy of a variety of nutrients into the readily available energy of adenosine triphosphate (ATP). This cycle is the "power plant" that energizes all metabolism and thus, life itself.
In aerobic organisms, the citric acid cycle is a metabolic pathway that forms part of the breakdown of carbohydrates, fats and proteins into carbon dioxide and water in order to generate energy. It is one of three metabolic pathways that are involved in fuel molecule catabolism and adenosine triphosphate production, the other two being glycolysis and oxidative phosphorylation. Glycolysis and oxidative phosphorylation are also tied to the citric acid cycle.
The citric acid cycle also provides precursors for many compounds, such as certain amino acids, and some of its reactions are important in cells performing fermentation reactions in the absence of oxygen.
This key metabolic cycle was established very early in the unfolding plan of creation as the molecules involved, and the set of enzymes that run the cycle, are essentially the same in all bacteria, fungi, plants, and animals. The implication is that the cycle was well established well before the last universal ancestor of all life. The current consensus is that this cycle predated the advent of free oxygen where it was "run in reverse" (energy was put into the cycle) to assemble important molecules.
The citric acid cycle is the focus of attention of both those advocating design by a supreme being and those opposing such design. Biochemist Michael Behe, in his 1996 book Darwin's Black Box, made the claim that Darwinian evolution cannot account for the biochemical complexity of the living cell, which thus must be the products of intelligent design. The essence of the argument is that aspects of cellular machinery (bacterial flagellum, blood clotting, cellular transport and immune systems, and metabolic pathways, etc.) are irreducibly complex, so that removal of any one part causes the system to break down. Thus, it is inconceivable how this could develop through natural selection. Those opposing Behe's thesis point to an paper by Melendez-Hevia, et al. (1996) purporting to present a feasible scenario for the evolution of the citric acid cycle from simpler biochemical systems.
The citric acid cycle is also known as the Krebs Cycle in honor of Sir Hans Adolf Krebs (1900 - 1981), who proposed the key elements of this pathway in 1937, and was awarded the Nobel Prize in Medicine for its discovery in 1953.
Basic process
In essence, the citric acid cycle plays a central role in the manipulation of small carbon-oxygen-hydrogen molecules. This cycle plays two key roles in metabolism.
Running in one direction, the cycle constructs many basic molecules on which the rest of metabolism is based. A metabolic process that builds larger molecules is called anabolism. Running in the opposite direction, the cycle combines small molecules with oxygen and captures the liberated energy to run all of metabolism, breaking down molecules into smaller units in the process. A metabolic process to break down molecules into smaller units is called catabolism. The citric acid cycle is considered an amphibolic pathway because it participates in both catabolism and anabolism.
In practice, a cell runs billions of such cycles simultaneously, most in the energy-generating direction. Bacterial prokaryotes run the cycle both ways in their cytoplasm. In eukaryote cells, such as in humans, this energy-generating cellular respiration is constrained to within the mitochondria, the bacteria-like powerhouses of the cell.
In oxygen-using aerobic organisms, the citric acid cycle is the second step in the breakdown of carbohydrates, fats, and proteins into carbon dioxide and water in order to generate energy. In essence, the citric acid cycle has food molecules fed into it by a preprocessing pathway. A basic food molecule, such as glucose, is first broken down, without oxygen, by a series of steps, into smaller molecules. Some energy is captured as a few ATP molecules during this preprocessing stage. In the absence of oxygen, no more energy can be extracted, and the waste is converted into molecules such as ethanol (alcohol) or lactic acid (involved in the cramp of a muscle cell). In aerobic organisms, the citric acid cycle and subsequent oxidative phosphorylation process generates a large number of ATP molecules.
In carbohydrate catabolism (the breakdown of sugars), the citric acid cycle follows glycolysis, which breaks down glucose (a six-carbon-molecule) into pyruvate (a three-carbon molecule). In eukaryotes, pyruvate moves into the mitochondria. It is converted into acetyl-CoA (acetyl coenzyme A) and enters the citric acid cycle.
In protein catabolism, proteins are broken down by protease enzymes into their constituent amino acids. These amino acids are brought into the cells and can be a source of energy by being funnelled into the citric acid cycle.
In fat catabolism, triglycerides are hydrolyzed to break them into fatty acids and glycerol. In the liver, the glycerol can be converted into glucose via dihydroxyacetone phosphate and glyceraldehyde-3-phosphate by way of gluconeogenesis (carbohydrate catabolism of the glucose can then take place, as above). In many tissues, especially heart tissue, fatty acids are broken down through a process known as beta oxidation, which results in acetyl-CoA that can be used in the citric acid cycle. Sometimes beta oxidation can yield propionyl CoA, which can result in further glucose production by gluconeogenesis in liver.
The citric acid cycle is always followed by oxidative phosphorylation. This process extracts the energy from NADH and FADH2, recreating NAD+ and FAD, so that the cycle can continue. The citric acid cycle itself does not use oxygen, but oxidative phosphorylation does.
The total energy gained from the complete breakdown of one molecule of glucose by glycolysis, the citric acid cycle, and oxidative phosphorylation equals about 36 ATP molecules.
The cycle continues, thousands of times a second. One turn of the cycle turns the glucose fragment into carbon dioxide and water, just as if it had burnt in a flame.
Location of cycle and inputs and outputs
The citric acid cycle takes place within the mitochondrial matrix in eukaryotes, and within the cytoplasm in prokaryotes. There are eight stages in the citric acid cycle.
Fuel molecule catabolism (including glycolysis) produces acetyl-CoA, a two-carbon acetyl group bound to coenzyme A. Acetyl-CoA is the main input to the citric acid cycle. Citrate is both the first and the last product of the cycle, and is regenerated by the condensation of oxaloacetate and acetyl-CoA.
A different enzyme catalyzes each of the eight stages in the citric acid cycle, meaning there are eight different enzymes used in the cycle.
| Molecule | Enzyme | Reaction type | Reactants/ Coenzymes |
Products/ Coenzymes |
|---|---|---|---|---|
| I. Citrate | 1. Aconitase | Dehydration | H2O | |
| II. cis-Aconitate | 2. Aconitase | Hydration | H2O | |
| III. Isocitrate | 3. Isocitrate dehydrogenase | Oxidation | NAD+ | NADH + H+ |
| IV. Oxalosuccinate | 4. Isocitrate dehydrogenase | Decarboxylation | ||
| V. α-Ketoglutarate | 5. α-Ketoglutarate dehydrogenase |
Oxidative decarboxylation |
NAD+ + CoA-SH |
NADH + H+ + CO2 |
| VI. Succinyl-CoA | 6. Succinyl-CoA synthetase | Hydrolysis | GDP + Pi |
GTP + CoA-SH |
| VII. Succinate | 7. Succinate dehydrogenase | Oxidation | FAD | FADH2 |
| VIII. Fumarate | 8. Fumarase | Addition (H2O) | H2O | |
| IX. L-Malate | 9. Malate dehydrogenase | Oxidation | NAD+ | NADH + H+ |
| X. Oxaloacetate | 10. Citrate synthase | Condensation | ||
| XI. Acetyl-CoA |
The sum of all reactions in the citric acid cycle is:
- Acetyl-CoA + 3 NAD+ + FAD + GDP + Pi + 3 H2O →
CoA-SH + 3 NADH + H+ + FADH2 + GTP + 2 CO2 + 3 H+
Two carbons are oxidized to CO2, and the energy from these reactions is stored in guanosine triphosphate (GTP), NADH and FADH2. NADH and FADH2 are coenzymes (molecules that enable or enhance enzymes) that store energy and are utilized in oxidative phosphorylation.
A simplified view of the process: The process begins with pyruvate, producing one CO2, then one CoA. It begins with the six carbon sugar, glucose. It produces 2 CO2 and consumes 3 NAD+ producing 3NADH and 3H+. It consumes 3 H2O and consumes one FAD, producing one FADH+.
Regulation
Many of the enzymes in the TCA cycle are regulated by negative feedback from ATP when the energy charge of the cell is high. Such enzymes include the pyruvate dehydrogenase complex that synthesises the acetyl-CoA needed for the first reaction of the TCA cycle. Also the enzymes citrate synthase, isocitrate dehydrogenase, and alpha-ketoglutarate dehydrogenase, which regulate the first three steps of the TCA cycle, are inhibited by high concentrations of ATP. This regulation ensures that the TCA cycle will not oxidise excessive amount of pyruvate and acetyl-CoA when ATP in the cell is plentiful. This type of negative regulation by ATP is by an allosteric mechanism. (Allosteric refers to the regulation of an enzyme or protein as a result of the binding of a molecule at a site other than the active site.)
Several enzymes are also negatively regulated when the level of reducing equivalents in a cell are high (high ratio of NADH/NAD+). This mechanism for regulation is due to substrate inhibition by NADH of the enzymes that use NAD+ as a substrate. This includes both the entry point enzymes pyruvate dehydrogenase and citrate synthase.
ReferencesISBN links support NWE through referral fees
- Behe, M. J. 1996. Darwin's Black Box. New York: The Free Press.
- Melendez-Hevia, E., Waddell, T. G., and Cascante, M. 1996. The puzzle of the citric acid cycle. Journal of Molecular Evolution 43:293-303.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.