Inorganic chemistry
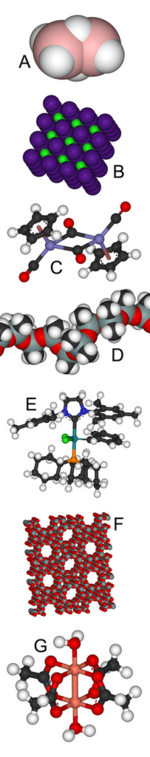
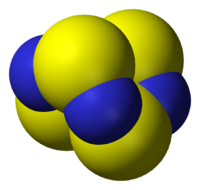
A: Diborane features unusual bonding.
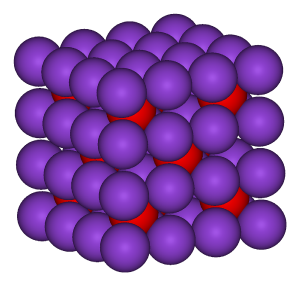
B: Caesium chloride has an archetypal crystal structure.
C: Fp2 is an organometallic complex.
D: Silicone's uses range from breast implants to Silly Putty.
E: Grubbs' catalyst, which led to the 2005 Nobel Prize in Chemistry for Robert H. Grubbs, its discoverer.
F: Zeolites find extensive use as molecular sieves.
G: Copper(II) acetate surprised theoreticians with its diamagnetism.
Inorganic chemistry is the branch of chemistry concerned with investigation of the properties of all elements, and the properties and methods of syntheses of their compounds, except for carbon and most carbon-containing compounds. (The study of some carbon-containing compounds—such as carbon dioxide, carbonates, and cyanides—is considered part of inorganic chemistry.) This field stands in a complementary relationship to organic chemistry, which covers the myriad carbon-based compounds. These two disciplines are generally considered separately, but there is much overlap, such as in the sub-discipline of organometallic chemistry.
Important classes of inorganic compounds include the oxides, sulfides, sulfates, carbonates, nitrates, and halides. Many of them are found in inanimate materials, such as minerals. For example, soil may contain iron sulfide as pyrite or calcium sulfate as gypsum. A number of inorganic compounds are found in biological systems, such as in the form of electrolytes (sodium chloride).
The study of inorganic chemistry has led to enormous benefits in practical terms. Traditionally, the scale of a nation's economy could be evaluated by its productivity of sulfuric acid. In 2005, the top 20 inorganic chemicals manufactured in Canada, China, Europe, Japan, and the United States were (in alphabetical order):[1] Aluminum sulfate, ammonia, ammonium nitrate, ammonium sulfate, carbon black, chlorine, hydrochloric acid, hydrogen, hydrogen peroxide, nitric acid, nitrogen, oxygen, phosphoric acid, sodium carbonate, sodium chlorate, sodium hydroxide, sodium silicate, sodium sulfate, sulfuric acid, and titanium dioxide.
Key concepts
Most inorganic compounds occur as salts, in which cations and anions are held together by ionic bonds. Examples of cations are sodium (Na+) and magnesium (Mg2+); examples of anions are oxide (O2−) and chloride (Cl−). These ions form compounds such as sodium oxide (Na2O) or magnesium chloride (MgCl2), which are neutral in their overall charge. The ions are described by their oxidation state and their ease of formation can be inferred from the ionization potential (for cations) or from the electron affinity (for anions) of the parent elements.
Many inorganic compounds are characterized by high melting points. Inorganic salts typically are poor conductors in the solid state. Other characteristic properties of inorganic compounds are their solubility in water (and other solvents) and ease of crystallization. Some compounds (such as sodium chloride, NaCl) are very soluble in water, others (such as silicon dioxide, SiO2) are not.
A simple inorganic reaction is double displacement, in which the ions of two salts are swapped without a change in oxidation state. In redox reactions, the oxidation state of one reactant, the oxidant, decreases, and that of the other reactant, the reductant, increases. The net result is an exchange of electrons. Electron exchange can occur indirectly as well, such as in electrical batteries—a key feature in electrochemistry.
Some inorganic compounds are acids or bases, and they undergo acid-base reactions. By the Brønsted-Lowry definition, an acid is a proton (hydrogen ion) donor; a base is a proton acceptor. By the Lewis definition, which is more general, any chemical species capable of binding to an electron pair is called a Lewis acid; conversely, any molecule that tends to donate an electron pair (to form a bond) is called a Lewis base.
The first important man-made inorganic compound was ammonium nitrite for soil fertilization through the Haber process. Inorganic compounds are synthesized for use as catalysts such as vanadium(V) oxide and titanium(III) chloride, or as reagents in organic chemistry such as lithium aluminum hydride.
Subdivisions of inorganic chemistry are organometallic chemistry, cluster chemistry, and bioinorganic chemistry. These fields are active areas research in inorganic chemistry, aimed toward new catalysts, superconductors, and therapies.
Descriptive inorganic chemistry
Descriptive inorganic chemistry focuses on the classification of compounds based on their properties. Partly the classification focuses on the position in the periodic table of the heaviest element (the element with the highest atomic weight) in the compound, partly by grouping compounds by their structural similarities. When studying inorganic compounds, one often encounters parts of the different classes of inorganic chemistry (an organometallic compound is characterized by its coordination chemistry, and may show interesting solid state properties).
Different classifications are:
Coordination compounds
Classical coordination compounds feature metals bound to "lone pairs" of electrons residing on the main group atoms of ligands such as H2O, NH3, Cl−, and CN−. In modern coordination, compounds almost all organic and inorganic compounds can be used as ligands. The "metal" usually is a metal from the groups 3-13, as well as the trans-lanthanides and trans-actinides, but from a certain perspective, all chemical compounds can be described as coordination complexes.
The stereochemistry of coordination complexes can be quite rich, as hinted at by Werner's separation of two enantiomers of [Co((OH)2Co(NH3)4)3]6+, an early demonstration that chirality is not inherent to organic compounds. A topical theme within this specialization is supramolecular coordination chemistry.[2]
- Examples: [Co(EDTA)]−, [Co(NH3)6]3+, TiCl4(THF)2.
Main group compounds
These species feature elements from groups 1, 2 and 13-18 (excluding hydrogen) of the periodic table. Due to their often similar reactivity, the elements in group 3 (Sc, Y, and La) and group 12 (Zn, Cd, and Hg) are also generally included.[3]
Main group compounds have been known since the beginnings of chemistry, for example, elemental sulfur and the distillable white phosphorus. Experiments on oxygen, O2, by Lavoisier and Priestley not only identified an important diatomic gas, but opened the way for describing compounds and reactions according to stoichiometric ratios. The discovery of a practical synthesis of ammonia using iron catalysts by Carl Bosch and Fritz Haber in the early 1900s deeply impacted mankind, demonstrating the significance of inorganic chemical synthesis. Typical main group compounds are SiO2, SnCl4, and N2O. Many main group compounds can also be classed as “organometallic,” as they contain organic groups, for example, B(CH3)3). Main group compounds also occur in nature, for example, phosphate in DNA, and therefore may be classed as bioinorganic. Conversely, organic compounds lacking (many) hydrogen ligands can be classed as “inorganic,” such as the fullerenes, buckytubes and binary carbon oxides.
- Examples: tetrasulfur tetranitride S4N4, diborane B2H6, silicones, buckminsterfullerene C60.
Transition metal compounds
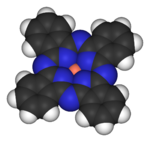
Compounds containing metals from group 4 to 11 are considered transition metal compounds. Compounds with a metal from group 3 or 12 are sometimes also incorporated into this group, but also often classified as main group compounds.
Transition metal compounds show a rich coordination chemistry, varying from tetrahedral for titanium (for example, TiCl4) to square planar for some nickel complexes to octahedral for coordination complexes of cobalt. A range of transition metals can be found in biologically important compounds, such as iron in hemoglobin.
- Examples: iron pentacarbonyl, titanium tetrachloride, cisplatin
Organometallic compounds
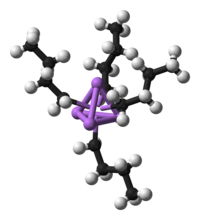
Usually, organometallic compounds are considered to contain the M-C-H group.[4] The metal (M) in these species can either be a main group element or a transition metal. Operationally, the definition of an organometallic compound is more relaxed to include also highly lipophilic complexes such as metal carbonyls and even metal alkoxides.
Organometallic compounds are mainly considered a special category because organic ligands are often sensitive to hydrolysis or oxidation, necessitating that organometallic chemistry employs more specialized preparative methods than was traditional in Werner-type complexes. Synthetic methodology, especially the ability to manipulate complexes in solvents of low coordinating power, enabled the exploration of very weakly coordinating ligands such as hydrocarbons, H2, and N2. Because the ligands are petrochemicals in some sense, the area of organometallic chemistry has greatly benefited from its relevance to industry.
- Examples: Cyclopentadienyliron dicarbonyl dimer (C5H5)Fe(CO)2CH3, Ferrocene Fe(C5H5)2, Molybdenum hexacarbonyl Mo(CO)6, Diborane B2H6, Tetrakis(triphenylphosphine)palladium(0) Pd[P(C6H5)3]4
Cluster compounds
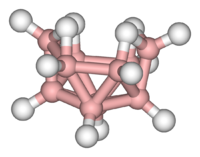
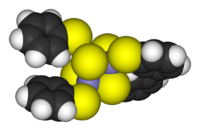
Clusters can be found in all classes of chemical compounds. According to the commonly accepted definition, a cluster consists minimally of a triangular set of atoms that are directly bonded to each other. But metal-metal bonded dimetallic complexes are highly relevant to the area. Clusters occur in "pure" inorganic systems, organometallic chemistry, main group chemistry, and bioinorganic chemistry. The distinction between very large clusters and bulk solids is increasingly blurred. This interface is the chemical basis of nanoscience or nanotechnology and specifically arise from the study of quantum size effects in cadmium selenide clusters. Thus, large clusters can be described as an array of bound atoms intermediate in character between a molecule and a solid.
- Examples: Fe3(CO)12, B10H14, [Mo6Cl14]2−, 4Fe-4S
Bioinorganic compounds
See also Bioorganometallic chemistry
These compounds occur (by definition) in nature, but the subfield includes anthropogenic species, such as pollutants and drugs, for example, Cisplatin.[5] The field includes many compounds, for example, the phosphates in DNA, but also metal complexes containing ligands that range from biological macromolecules, commonly peptides, to ill-defined species such as humic acid, and to water (for example, coordinated to gadolinium complexes employed for MRI).
- Examples: hemoglobin, methylmercury, carboxypeptidase
Solid state compounds
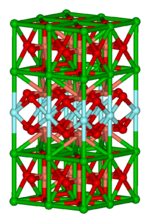
This important area focuses on structure,[6] bonding, and the physical properties of materials. In practice, solid state inorganic chemistry uses techniques such as crystallography to gain an understanding of the properties that result from collective interactions between the subunits of the solid. Included in solid state chemistry are metals and their alloys or intermetallic derivatives. Related fields are condensed matter physics, mineralogy, and materials science.
- Examples: silicon chips, zeolites, YBa2Cu3O7
Theoretical inorganic chemistry
An alternative perspective on the area of inorganic chemistry begins with the Bohr model of the atom and, using the tools and models of theoretical chemistry and computational chemistry, expands into bonding in simple and then more complex molecules. Precise quantum mechanical descriptions for multielectron species, the province of inorganic chemistry, is difficult. This challenge has spawned many semi-quantitative or semi-empirical approaches including molecular orbital theory and ligand field theory, In parallel with these theoretical descriptions, approximate methodologies are employed, including density functional theory.
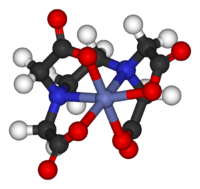
Exceptions to theories, qualitative and quantitative, are extremely important in the development of the field. For example, CuII2(OAc)4(H2O)2 is almost diamagnetic below room temperature whereas Crystal Field Theory predicts that the molecule would have two unpaired electrons. The disagreement between qualitative theory (paramagnetic) and observation (diamagnetic) led to the development of models for "magnetic coupling." These improved models led to the development of new magnetic materials and new technologies.
Qualitative theories
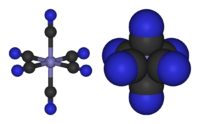
Inorganic chemistry has greatly benefited from qualitative theories. Such theories are easier to learn as they require little background in quantum theory. Within main group compounds, VSEPR theory powerfully predicts, or at least rationalizes, the structures of main group compounds, such as an explanation for why NH3 is pyramidal whereas ClF3 is T-shaped. For the transition metals, crystal field theory allows one to understand the magnetism of many simple complexes, such as why [FeIII(CN)6]3− has only one unpaired electron, whereas [FeIII(H2O)6]3+ has five. A particularly powerful qualitative approach to assessing the structure and reactivity begins with classifying molecules according to electron counting, focusing on the numbers of valence electrons, usually at the central atom in a molecule.
Group Theory
A central construct in inorganic chemistry is Group Theory.[7] Group Theory provides the language to describe the shapes of molecules according to their "point group symmetry." Group Theory also enables factoring and simplification of theoretical calculations.
Spectroscopic features are analyzed and described with respect to the symmetry properties of the, inter alia, vibrational or electronic states. Knowledge of the symmetry properties of the ground and excited states allows one to predict the numbers and intensities of absorptions in vibrational and electronic spectra. A classic application of Group Theory is the prediction of the number of C-O vibrations in substituted metal carbonyl complexes. The most common applications of symmetry to spectroscopy involve vibrational and electronic spectra.
As an instructional tool, Group Theory highlights commonalities and differences in the bonding otherwise disparate species, such as WF6 and Mo(CO)6 or CO2 and NO2.
Reaction pathways
The theory of chemical reactions is more challenging than the theory for a static molecule. Marcus theory provides a powerful linkage between bonding, mechanism, and reactivity. The relative strengths of metal-ligand bonds, which can be calculated theoretically, anticipates the kinetically accessible pathways.
Thermodynamics and inorganic chemistry
An alternative quantitative approach to inorganic chemistry focuses on energies of reactions. This approach is highly traditional and empirical, but it is also useful. Broad concepts that are couched in thermodynamic terms include redox potential, acidity, phase changes. A classic concept in inorganic thermodynamics is the Born-Haber cycle, which is used for assessing the energies of elementary processes such as electron affinity, some of which cannot be observed directly.
Mechanistic inorganic chemistry
An important and increasingly popular aspect of inorganic chemistry focuses on reaction pathways. The mechanisms of reactions are discussed differently for different classes of compounds.
Main group elements and lanthanides
The mechanisms of main group compounds of groups 13-18 are usually discussed in the context of organic chemistry (organic compounds are main group compounds, after all). Elements heavier than C, N, O, and F often form compounds with more electrons than predicted by the octet rule, as explained in the article on hypervalent molecules. The mechanisms of their reactions differ from organic compounds for this reason. Elements lighter than carbon (B, Be, Li) as well as Al and Mg often form electron-deficient structures that are electronically akin to carbocations. Such electron-deficient species tend ro react via associative pathways. The chemistry of the lanthanides mirrors many aspects of chemistry seen for aluminium.
Transition metal complexes
Mechanisms for the reactions of transition metals are discussed differently from main group compounds.[8] The important role of d-orbitals in bonding strongly influences the pathways and rates of ligand substitution and dissociation. These themes are covered in articles on coordination chemistry and ligand. Both associative and dissociative pathways are observed.
An overarching aspect of mechanistic transition metal chemistry is the kinetic lability of the complex illustrated by the exchange of free and bound water in the prototypical complexes [M(H2O)6]n+:
- [M(H2O)6]n+ + 6 H2O* → [M(H2O*)6]n+ + 6 H2O
- where H2O* denotes isotopically enriched water, e.g. H217O
The rates of water exchange varies by 20 orders of magnitude across the periodic table, with lanthanide complexes at one extreme and Ir(III) species being the slowest.
Redox reactions
Redox reactions are prevalent for the transition elements. Two classes of redox reaction are considered: Atom-transfer reactions, such as oxidative addition/reductive elimination, and electron-transfer. A fundamental redox reaction is "self-exchange," which involves the degenerate reaction between an oxidant and a reductant. For example, permanganate and its one-electron reduced relative manganate exchange one electron:
- [MnO4]− + [Mn*O4]2− → [MnO4]2− + [Mn*O4]−
Reactions at ligands
Coordinated ligands display reactivity distinct from the free ligands. For example, the acidity of the ammonia ligands in [Co(NH3)6]3+ is elevated relative to NH3 itself. Alkenes bound to metal cations are reactive toward nucleophiles whereas alkenes normally are not. The large and industrially important area of catalysis hinges on the ability of metals to modify the reactivity of organic ligands. Homogeneous catalysis occurs in solution and heterogeneous catalysis occurs when gaseous or dissolved substrates interact with surfaces of solids. Traditionally homogeneous catalysis is considered part of organometallic chemistry and heterogeneous catalysis is discussed in the context of surface science, a subfield of solid state chemistry. But the basic inorganic chemical principles are the same. Transition metals, almost uniquely, react with small molecules such as CO, H2, O2, and C2H4. The industrial significance of these feedstocks drives the active area of catalysis.
Characterization of inorganic compounds
Because of the diverse range of elements and the correspondingly diverse properties of the resulting derivatives, inorganic chemistry is closely associated with many methods of analysis. Older methods tended to examine bulk properties such as the electrical conductivity of solutions, melting points, solubility, and acidity. With the advent of quantum theory and the corresponding expansion of electronic apparatus, new tools have been introduced to probe the electronic properties of inorganic molecules and solids. Often these measurements provide insights relevant to theoretical models. For example, measurements on the photoelectron spectrum of methane demonstrated that describing the bonding by the two-center, two-electron bonds predicted between the carbon and hydrogen using Valence Bond Theory is not appropriate for describing ionization processes in a simple way. Such insights led to the popularization of molecular orbital theory as fully delocalized orbitals are a more appropriate simple description of electron removal and electron excitation.
Commonly encountered techniques are:
- X-ray crystallography: This technique allows for the 3D determination of molecular structures.
- Spectroscopy: Historically, UV-vis spectroscopy has been an important tool, since many inorganic compounds are strongly colored.
- Electron-spin resonance: ESR (or EPR) allows for the measurement of the environment of paramagnetic metal centra.
- Electrochemistry: Cyclic voltammetry and related techniques probe the redox characteristics of compounds.
- NMR spectroscopy: Besides 1H and 13C many other "good" NMR nuclei (for example, 11B, 19F, 31P, and 195Pt) give important information on compound properties and structure. Also the NMR of paramagnetic species can result in important structural information.
- Electron-nuclear double resonance (ENDOR) spectroscopy
- Mössbauer spectroscopy
Synthetic inorganic chemistry
Although some inorganic species can be obtained in pure form from nature, most are synthesized in chemical plants and in the laboratory.
Inorganic synthetic methods can be classified roughly according the volatility or solubility of the component reactants.[9] Soluble inorganic compounds are prepared using methods of organic synthesis. For metal-containing compounds that are reactive toward air, Schlenk line and glove box techniques are followed. Volatile compounds and gases are manipulated in “vacuum manifolds” consisting of glass piping interconnected through valves, the entirety of which can be evacuated to 0.001 mm Hg or less. Compounds are condensed using liquid nitrogen (b.p. 78K) or other cryogens. Solids are typically prepared using tube furnaces, the reactants and products being sealed in containers, often made of fused silica (amorphous SiO2) but sometimes more specialized materials such as welded Ta tubes or Pt “boats.” Products and reactants are transported between temperature zones to drive reactions.
See also
- Chemical compound
- Chemical element
- Chemical formula
- Chemical substance
- Chemistry
- Organic chemistry
Notes
- ↑ "Facts & Figures Of The Chemical Industry," Chemical and Engineering News July 10, 2006.
- ↑ J.M. Lehn, Supramolecular Chemistry: Concepts and Perspectives (VCH: Weinhiem, 1995).
- ↑ N.N. Greenwood & A. Earnshaw, Chemistry of the Elements, 2nd Ed (Oxford: Butterworth-Heinemann, 1999, ISBN 0-7506-3365-4).
- ↑ C. Elschenbroich, A. Salzer, Organometallics : A Concise Introduction 2nd Ed (Wiley-VCH: Weinheim, 1992, ISBN 3-527-28165-7).
- ↑ S. J. Lippard, J. M. Berg, Principles of Bioinorganic Chemistry (Mill Valley, CA: University Science Books, 1994, ISBN 0-935702-73-3).
- ↑ A.F. Wells, Structural Inorganic Chemistry (Oxford: Clarendon Press, 1984).
- ↑ F.A. Cotton, Chemical Applications of Group Theory (New York: John Wiley & Sons, 1990).
- ↑ R. G. Wilkins, Kinetics and Mechanism of Reactions of Transition Metal Complexes (Wiley-VCH Verlag, 1991, ISBN 3-527-28389-7).
- ↑ G.S. Girolami, T.B. Rauchfuss, and R.J. Angelici, Synthesis and Technique in Inorganic Chemistry (Mill Valley, CA: University Science Books, 1999).
ReferencesISBN links support NWE through referral fees
- Brown Jr., Theodore L., H. Eugene LeMay, Bruce Edward Bursten, and Julia R. Burdge. 2002. Chemistry: The Central Science, 9th ed. Upper Saddle River, NJ: Prentice Hall. ISBN 0130669970.
- Chang, Raymond. 2006. Chemistry, 9th ed. New York, NY: McGraw-Hill Science/Engineering/Math. ISBN 0073221031.
- Cotton, F. Albert, and Geoffrey Wilkinson. 1980. Advanced Inorganic Chemistry, 4th ed. New York, NY: Wiley. ISBN 0-471-02775-8.
- Greenwood, N.N., and A. Earnshaw. 1998. Chemistry of the Elements, 2nd ed. Oxford, U.K.; Burlington, MA: Butterworth-Heinemann, Elsevier Science. ISBN 0750633654.
- Moore, John W., Conrad L. Stanitski, and Peter C. Jurs. 2005. Chemistry: The Molecular Science, 2nd ed. Belmont, CA: Thompson Brooks/Cole. ISBN 9780534422011.
| ||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.