Functional group
In organic chemistry, functional groups (or moieties) are specific groups of atoms within molecules, that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction(s) regardless of the size of the molecule it is a part of.
Combining the names of functional groups with the names of the parent alkanes generates a powerful systematic nomenclature for naming organic compounds.
The non-hydrogen atoms of functional groups are always associated with each other and with the rest of the molecule by covalent bonds. When the group of atoms is associated with the rest of the molecule primarily by ionic forces, the group is referred to more properly as a polyatomic ion or complex ion—all of these are called radicals, by a meaning of the term radical that predates the free radical.
The first carbon atom after the carbon that attaches to the functional group is called the alpha carbon.
Functional groups are attached to the carbon backbone of organic molecules. They determine the characteristics and chemical reactivity of molecules. Functional groups are far less stable than the carbon backbone and are likely to participate in chemical reactions.
Table of common functional groups
The following is a list of common functional groups. In the formulas, the symbols R and R' usually denotes an attached hydrogen, or a hydrocarbon side chain of any length, but may sometimes refer to any group of atoms. Below is an image of multiple functional groups found in organic chemistry.
(For convenience, see basic functional groups covered in General Biology)
| Chemical class | Group | Formula | Structural Formula | Prefix | Suffix | Example |
|---|---|---|---|---|---|---|
| Acyl halide | Haloformyl | RCOX | 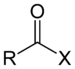
|
haloformyl- | -oyl halide | 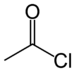 Acetyl chloride (Ethanoyl chloride) |
| Alcohol | Hydroxyl | ROH | 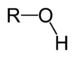
|
hydroxy- | -ol | 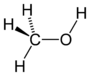 Methanol |
| Aldehyde | Aldehyde | RCHO | 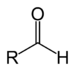
|
aldo- | -al | 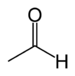 Acetaldehyde (Ethanal) |
| Alkane | Alkyl | RH | alkyl- | -ane | 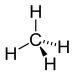 Methane | |
| Alkene | Alkenyl | R2C=CR2 | alkenyl- | -ene | 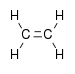 Ethylene (Ethene) | |
| Alkyne | Alkynyl | RC≡CR' | alkynyl- | -yne | ||
| Amide | Carboxamide | RCONR2 | 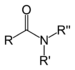
|
carboxamido- | -amide | |
| Amines | Primary amine | RNH2 | amino- | -amine | 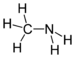 Methylamine (Methanamine) | |
| Secondary amine | R2NH | 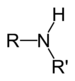
|
amino- | -amine | Dimethylamine | |
| Tertiary amine | R3N | 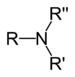
|
amino- | -amine | 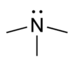 Trimethylamine | |
| 4° ammonium ion | R4N+ | 
|
ammonio- | -ammonium | 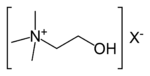 Choline | |
| Azide | Azide | RN3 | azido- | alkyl azide | 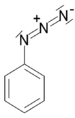 Phenyl azide (Azidobenzene) | |
| Azo compound | Azo (Diimide) |
RN2R' | 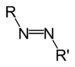
|
azo- | -diazene | 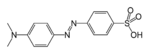 Methyl orange (p-dimethylamino-azobenzenesulfonic acid) |
| Toluene derivative | Benzyl | RCH2C6H5 RBn |
benzyl- | 1-(substituent)toluene | Benzyl bromide (1-Bromotoluene) | |
| Carbonate | Carbonate ester | ROCOOR | alkyl carbonate | |||
| Carboxylate | Carboxylate | RCOO− | 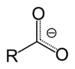 |
carboxy- | -oate | Sodium acetate (Sodium ethanoate) |
| Carboxylic acid | Carboxyl | RCOOH | 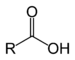
|
carboxy- | -oic acid | |
| Cyanates | Cyanate | ROCN | cyanato- | alkyl cyanate | ||
| Thiocyanate | RSCN | thiocyanato- | alkyl thiocyanate | |||
| Disulfide | Disulfide | RSSR' | alkyl alkyl disulfide | Cystamine (2,2'-Dithiobis(ethylamine)) | ||
| Ether | Ether | ROR' | alkoxy- | alkyl alkyl ether | Diethyl ether (Ethoxyethane) | |
| Ester | Ester | RCOOR' | 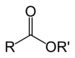
|
alkyl alkanoate | Ethyl butyrate (Ethyl butanoate) | |
| Haloalkane | Halo | RX | halo- | alkyl halide | Chloroethane (Ethyl chloride) | |
| Hydroperoxide | Hydroperoxy | ROOH | hydroperoxy- | alkyl hydroperoxide | Methyl ethyl ketone peroxide | |
| Imine | Primary ketimine | RC(=NH)R' | 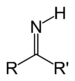
|
imino- | -imine | |
| Secondary ketimine | RC(=NR)R' | 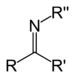
|
imino- | -imine | ||
| Primary aldimine | RC(=NH)H | 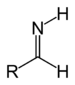
|
imino- | -imine | ||
| Secondary aldimine | RC(=NR')H | 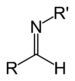
|
imino- | -imine | ||
| Imide | Imide | RC(=O)NC(=O)R' | 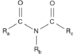
|
imido- | -imide | |
| Isocyanide | Isocyanide | RNC | isocyano- | alkyl isocyanide | ||
| Isocyanates | Isocyanate | RNCO | isocyanato- | alkyl isocyanate | Methyl isocyanate | |
| Isothiocyanate | RNCS | isothiocyanato- | alkyl isothiocyanate | Allyl isothiocyanate | ||
| Ketone | Carbonyl | RCOR' | 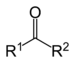
|
keto-, oxo- | -one | 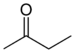 Methyl ethyl ketone (Butanone) |
| Nitrate | Nitrate | RONO2 | nitrooxy-, nitroxy- |
alkyl nitrate |
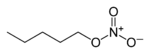 Amyl nitrate (1-nitrooxypentane) | |
| Nitrile | Nitrile | RCN | cyano- |
alkanenitrile |
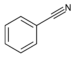 Benzonitrile (Phenyl cyanide) | |
| Nitrite | Nitrosooxy | RONO | nitrosooxy- |
alkyl nitrite |
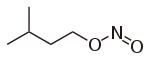 Amyl nitrite (3-methyl-1-nitrosooxybutane) | |
| Nitro compound | Nitro | RNO2 | 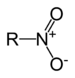
|
nitro- | 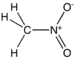 Nitromethane | |
| Nitroso compound | Nitroso | RNO | nitroso- | Nitrosobenzene | ||
| Peroxide | Peroxy | ROOR | 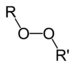
|
peroxy- | alkyl peroxide | Di-tert-butyl peroxide |
| Benzene derivative | Phenyl | RC6H5 | phenyl- | -benzene | 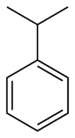 Cumene (2-phenylpropane) | |
| Phosphine | Phosphino | R3P | 
|
phosphino- | -phosphane | Methylpropylphosphane |
| Phosphodiester | Phosphate | HOPO(OR)2 | 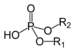
|
phosphoric acid di(substituent) ester | di(substituent) hydrogenphosphate | DNA |
| Phosphonic acid | Phosphono | RP(=O)(OH)2 | 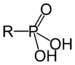
|
phosphono- | substituent phosphonic acid | Benzylphosphonic acid |
| Phosphate | Phosphate | ROP(=O)(OH)2 | 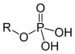
|
phospho- | Glyceraldehyde 3-phosphate | |
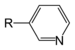
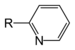
| Pyridine derivative | Pyridyl | RC5H4N |
4-pyridyl |
-pyridine | 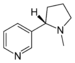 Nicotine | |
| Sulfide | RSR' | di(substituent) sulfide | Dimethyl sulfide | |||
| Sulfone | Sulfonyl | RSO2R' | 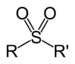
|
sulfonyl- | di(substituent) sulfone | 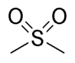 Dimethyl sulfone (Methylsulfonylmethane) |
| Sulfonic acid | Sulfo | RSO3H | 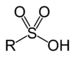
|
sulfo- | substituent sulfonic acid | 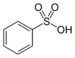 Benzenesulfonic acid |
| Sulfoxide | Sulfinyl | RSOR' | 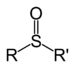
|
sulfinyl- | di(substituent) sulfoxide | 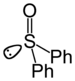 Diphenyl sulfoxide |
| Thiol | Sulfhydryl | RSH | 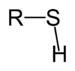
|
mercapto-, sulfanyl- | -thiol | Ethanethiol (Ethyl mercaptan) |
See also
- Chemical classification
- Organic chemistry
ReferencesISBN links support NWE through referral fees
- McMurry, John. Organic Chemistry. 6th ed. Belmont, CA: Brooks/Cole, 2004. ISBN 0534420052
- Morrison, Robert T., and Robert N. Boyd. Organic Chemistry. 6th ed. Englewood Cliffs, NJ: Prentice Hall, 1992. ISBN 0-13-643669-2
- Solomons, T.W. Graham, and Fryhle, Craig B. Organic Chemistry. 8th ed. Hoboken, NJ: John Wiley, 2004. ISBN 0471417998
External links
All links retrieved April 15, 2024.
- IUPAC Compendium of Chemical Terminology - the Gold Book International Union of Pure and Applied Chemistry.
| Functional groups |
|---|
| Chemical class: Alcohol • Aldehyde • Alkane • Alkene • Alkyne • Amide • Amine • Azo compound • Benzene derivative • Carboxylic acid • Cyanate • Ester • Ether • Haloalkane • Imine • Isocyanide • Isocyanate • Ketone • Nitrile • Nitro compound • Nitroso compound • Peroxide • Phosphoric acid • Pyridine derivative • Sulfone • Sulfonic acid • Sulfoxide • Thioether • Thiol • Toluene derivative |
| Topics in organic chemistry |
|---|
|
Aromaticity | Covalent bonding | Functional groups | Nomenclature | Organic compounds | Organic reactions | Organic synthesis | Publications | Spectroscopy | Stereochemistry |
| List of organic compounds |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.