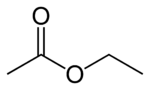
Ethyl acetate
| Ethyl acetate | |
|---|---|
| |
| General | |
| IUPAC name | Ethyl acetate |
| Systematic name | Ethyl ethanoate |
| Other names | ethyl ester, ethyl acetate, acetic ester, ester of ethanol |
| Molecular formula | C4H8O2 |
| SMILES | CCOC(C)=O |
| Molar mass | 88.105 g/mol |
| Appearance | colorless liquid |
| CAS number | [141-78-6] |
| Properties | |
| Density and phase | 0.897 g/cm³, liquid |
| Solubility in water | 8.3 g/100 mL (20 °C) |
| Solubility in ethanol, acetone, diethyl ether, benzene |
Miscible |
| Melting point | −83.6 °C (189.55 K) |
| Boiling point | 77.1 °C (350.25 K) |
| Critical temperature | 250.11 °C (523.26 K) |
| Viscosity | 0.426 cP at 25 °C |
| Structure | |
| Dipole moment | 1.78 D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | Flammable (F), Irritant (Xi) |
| NFPA 704 | |
| R-phrases | R11, R36, R66, R67 |
| S-phrases | S16, S26, S33 |
| Flash point | −4 °C |
| RTECS number | AH5425000 |
| Supplementary data page | |
| Structure and properties |
n = 1.3720 |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Related carboxylate esters | Methyl acetate, Butyl acetate |
| Related compounds | Acetic acid, ethanol |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Ethyl acetate is an organic compound that is an ester derived from the combination of ethanol and acetic acid. Its chemical formula may be written as CH3CH2OC(O)CH3 or CH3CO2C2H5, and chemists often abbreviate its name as EtOAc. It is a colorless liquid with a characteristic smell that is slightly sweet and fruity.
Ethyl acetate is manufactured on a large scale for use as a solvent, such as for nail polish and nail polish removers. It is also useful in the process of decaffeination of coffee and tea. It is an ingredient in confectionery and perfumes, and it is added to paints to serve as an activator or hardener. Entomologists use it for insect collection, preservation, and study.
Occurrence in nature
Ethyl acetate is a by-product of fermentation and is present in fruits and wines. At low concentrations, it can enhance the taste of a wine, but it is considered a contaminant at relatively high concentrations, as occurs when wine is exposed to air for a prolonged period. When present at too high a concentration in wine, it is regarded as an off-flavor.
Properties
Ethyl acetate is a moderately polar solvent that has the advantages of being volatile, relatively non-toxic, and non-hygroscopic. It is a weak hydrogen bond acceptor, and is not a donor due to the lack of an acidic proton (that is, a hydrogen atom directly bonded to an electronegative atom such as fluorine, oxygen, or nitrogen). Ethyl acetate can dissolve up to three percent water and has a solubility of eight percent in water at room temperature. At elevated temperature its solubility in water is higher.
Reactions
Ethyl acetate can be hydrolyzed in acidic or basic conditions to produce acetic acid and ethanol. However, the use of an acid catalyst (such as sulfuric acid) gives poor yields, because the forward reaction is in equilibrium with the backward reaction.
To obtain high yields of the products, it is preferable to use a stoichiometric amount of strong base, such as sodium hydroxide. This reaction gives ethanol and sodium acetate, which is not able to react with ethanol any longer. The reaction may be written as:
- CH3CO2C2H5 + NaOH → C2H5OH + CH3CO2Na
Synthesis
Ethyl acetate is synthesized via the Fischer esterification reaction from acetic acid and ethanol, typically in the presence of an acid catalyst such as sulfuric acid.
- CH3CH2OH + CH3COOH → CH3COOCH2CH3 + H2O
Because the reaction is reversible and produces an equilibrium, the yield is low unless water is removed. In the laboratory, the ethyl acetate product can be isolated from water using what is called a Dean-Stark apparatus.
Uses
- Ethyl acetate is widely employed as a solvent for nail varnishes and nail varnish removers.
- Industrially, it is used to decaffeinate coffee beans and tea leaves.
- In chemistry, it is often mixed with a non-polar solvent such as hexanes as a chromatography solvent. It is also used as a solvent for extractions.
- It is used in confectionery and perfumes. It is used in perfumes because it confers a fruity smell (as do many esters) and evaporates quickly, leaving the scent of the perfume on the skin.
- It is used in paints as an activator or hardener.
- In the field of entomology, ethyl acetate is an effective poison for use in insect collecting and study. In a killing jar charged with ethyl acetate, the vapors will kill the collected (usually adult) insect quickly without destroying it. Because it is not hygroscopic, ethyl acetate also keeps the insect soft enough to allow proper mounting suitable for a collection.
See also
ReferencesISBN links support NWE through referral fees
- McMurry, John. Organic Chemistry, 6th ed. Belmont, CA: Brooks/Cole, 2004. ISBN 0534420052
- Morrison, Robert T., and Robert N. Boyd. Organic Chemistry, 6th ed. Englewood Cliffs, NJ: Prentice Hall, 1992. ISBN 0-13-643669-2
- Solomons, T.W. Graham, and Craig B. Fryhle. Organic Chemistry, 8th ed. Hoboken, NJ: John Wiley, 2004. ISBN 0471417998
External links
All links retrieved March 22, 2024.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
