Chromatography
Chromatography (from Greek χρώμα chroma, meaning "color") is the collective term for a family of laboratory techniques for the separation of mixtures. Basically, its a group of different methods used to separate or analyze mixtures. It involves passing a mixture through a stationary phase, which separates the analyte to be measured from other molecules in the mixture and allows it to be isolated.
The main use of chromatography is to purify a certain material from a mixture. It is so precise that it can be used either to separate proteins that only vary by one amino acid as well as to purify volatile or soluble material.
Types
Different types of Chormatography and some uses are:
- Liquid Chromatography—used to analyze water samples to look for pollution, metal ions, organic compounds. Liquid is used to mesh hydrophilic and insoluble molecules
- Paper Chromatography—most common method which uses paper and through capillary action, solvents are pulled up and separated
- Gas Chromatography—used in forensics in analyzing fibers, blood. Helium is used to move a gas mixture through a column
- Thin Layer Chromatography—checks purity of compounds, such as pestisides or substances in food. TLC uses absorbent material on flat glass or plastic plates
History
Chromatography means "to show with colors." It was the Russian botanist Mikhail Semyonovich Tsvet (1872-1919) who invented the first chromatography technique in 1900, during his research on chlorophyll. He used a liquid-adsorption column containing calcium carbonate to separate plant pigments. The method was described on December 30, 1901, at the 11th Congress of Naturalists and Doctors (XI съезд естествоиспытателей и врачей) in St. Petersburg. The first printed description was in 1903, in the Proceedings of the Warsaw Society of Naturalists, section of biology. He first used the term "chromatography" in print in 1906, in his two papers about chlorophyll in the German botanical journal, Berichte der Deutschen Botanischen Gesellschaft. In 1907, he demonstrated his chromatograph for the German Botanical Society. Interestingly, Mikhail's surname "Tsvet" means "color" in Russian, so some have suggested that he named the procedure chromatography (literally "color writing") to ensure that he, a commoner in Tsarist Russia, would be remembered for his work.
In 1952, Archer John Porter Martin and Richard Laurence Millington Synge were awarded the Nobel Prize in Chemistry for their invention of partition chromatography.[1] Since then, the technology has advanced rapidly. Researchers found that the principles underlying Tsvet's chromatography could be applied in many different ways, giving rise to the different varieties of chromatography described below. Simultaneously, advances continually improved the technical performance of chromatography, allowing the separation of increasingly similar molecules.
Chromatography terms
- The analyte is the substance which is to be purified or isolated during chromatography
- Analytical chromatography is used to determine the identity and concentration of molecules in a mixture
- A chromatogram is the visual output of the chromatograph. Different peaks or patterns on the chromatogram correspond to different components of the separated mixture
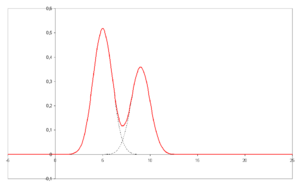
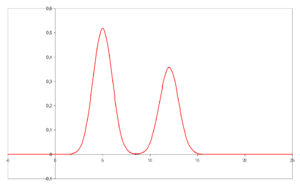
- Plotted on the x-axis is the retention time and plotted on the y-axis a signal (for example obtained by UV spectroscopy) corresponding to the amount of analyte exiting the system.
- A chromatograph takes a chemical mixture carried by liquid or gas and separates it into its component parts as a result of differential distributions of the solutes as they flow around or over the stationary phase
- The mobile phase is the analyte and solvent mixture which travels through the stationary phase
- Preparative chromatography is used to nondestructively purify sufficient quantities of a substance for further use, rather than analysis.
- The retention time is the characteristic time it takes for a particular molecule to pass through the system under set conditions.
- The stationary phase is the substance which is fixed in place for the chromatography procedure and is the phase to which solvents and the analyte travels through or binds to. Examples include the silica layer in thin layer chromatography.
- The bonded phase is the phase which is covalently bonded to the support particles or to the inside wall of the column tubing.
- The Column Length is proportional to the number of theoretical plates. Shorter columns show higher resolutions.
Chromatography theory
Chromatography is a separation method that defines the differences in partitioning behavior between a mobile phase and a stationary phase to separate the components in a mixture. Components of a mixture may be interacting with the stationary phase based on charge, relative solubility or adsorption. There are two theories of chromatography, the plate and rate theories.
Retention
The retention is a measure of the speed at which a substance moves in a chromatographic system. The retention volume of a solute is that volume of mobile phase that passes through the column between the injection point and the peak maximum.In continuous development systems like HPLC or GC, where the compounds are eluted with the eluent, the retention is usually measured as the retention time Rt or tR, the time between injection and detection. In interrupted development systems like TLC the retention is measured as the retention factor Rf, the run length of the compound divided by the run length of the eluent front:
The retention of a compound often differs considerably between experiments and laboratories due to variations of the eluent, the stationary phase, temperature, and the setup. It is therefore important to compare the retention of the test compound to that of one or more standard compounds under absolutely identical conditions.
During the chromatographic process the analyte experiences zone broadening as a result of diffusion. Two analytes with different retention times yet with large broadening do not resolve and this is why in any chromatographic system broadening needs to be minimized. This is done by selecting the proper stationary and mobile phase, the eluent velocity, the track length and temperature. The Van Deemter's equation gives an ideal eluent velocity taking into account several physical parameters.
Plate theory
The plate theory of chromatography was developed by Archer John Porter Martin and Richard Laurence Millington Synge. They stated that each plate should be a broken into a specific length, and the solute spends a finite (limited) amount of time in each place. The size of the cell is there equilibriate between the two phases. The smaller the plate, the faster the equilibrium and the more plate in the column. This is further related to column efficiency.
The plate theory describes the chromatography system, the mobile and stationary phases, as being in equilibrium. The partition coefficient K is based on this equilibrium, and is defined by the following equation:
K is assumed to be independent of concentration, and can change if experimental conditions are changed, for example temperature is increased or decreased. As K increases, it takes longer for solutes to separate. For a column of fixed length and flow, the retention time and retention volume can be measured and used to calculate K.
Capillary-action chromatography

Paper chromatography
This old technique is used to analyze complex mixtures, such as ink, by separating the different chemicals it was made from. The method involves placing a small spot of sample solution onto a strip of chromatography paper. The paper is placed into a jar containing a shallow layer of solvent and sealed. As the solvent rises through the paper it meets the sample mixture which starts to travel up the paper with the solvent. Different compounds in the sample mixture travel different distances according to how strongly they interact with the paper. Mixtures of different characteristics (size and solubility) travel at different speeds. This allows the calculation of an Rf value and can be compared to standard compounds to aid in the identification of an unknown substance.
Thin layer chromatography
Thin layer chromatography (TLC) is a widely-employed laboratory technique and is similar to paper chromatography. However, instead of using a stationary phase of paper, it involves a stationary phase of a thin layer of adsorbent like silica gel, alumina, or cellulose on a flat, inert substrate. Compared to paper, it has the advantage of faster runs, better separations, and the choice between different adsorbents. Different compounds in the sample mixture travel different distances according to how strongly they interact with the adsorbent. This allows the calculation of an Rf value and can be compared to standard compounds to aid in the identification of an unknown substance.
Column chromatography
Column chromatography encompasses a number of techniques based around utilizing a vertical glass column filled with some form of solid adsorbent, with the sample to be separated placed on top of this support. The top is then filled with a liquid, which flows down through the column. Similarly to other forms of chromatography, differences in rates of movement through the solid medium are translated to different exit times from the bottom of the column for the various elements of the original sample. If the solvent moves down by gravity, it is gravity column chromatography. If the solvent moves down by air pressure, it is known as flash chromatography.
Flash chromatography
In 1978, W.C. Still introduced a modified version of column chromatography called flash column chromatography (flash).[2] The technique is very similar to the traditional column chromatography, except that the solvent is driven through the column by applying positive pressure. This method is used solely in organic teaching labs because of its ease and environmentally friendly nature. This allowed most separations to be performed in less than 20 minutes, with improved separations compared to the old method. Modern flash chromatography systems are sold as pre-packed plastic cartridges, and the solvent is pumped through the cartridge. Systems may also be linked with detectors and fraction collectors providing automation. The introduction of gradient pumps resulted in quicker separations and less solvent usage.
Fast performance liquid chromatography
Fast performance liquid chromatography (FPLC) is a term applied to several chromatography techniques which are used to purify proteins. Many of these techniques are identical to those carried out under high performance liquid chromatography.
FPLC involves using a pump and column which withstand high pressure so separations are quickly induced.
High performance liquid chromatography
High performance liquid chromatography (HPLC) is a form of column chromatography used frequently in biochemistry and analytical chemistry. The analyte is forced through a column (stationary phase) by a liquid (mobile phase) at high pressure, which decreases the time the separated components remain on the stationary phase and thus the time they have to diffuse within the column.
Specific techniques which come under this broad heading are listed below. It should also be noted that the following techniques can also be considered fast protein liquid chromatography if no pressure is used to drive the mobile phase through the stationary phase. It is not dissimilar from Aqueous Normal Phase Chromatography.
Ion exchange chromatography
Ion exchange chromatography (IEC) is a column chromatography based on charge. It is used to separate charged compounds including amino acids, peptides, and proteins. The stationary phase is usually an ion exchange resin that carries charged functional groups which interact with oppositely charged groups of the compound to be retained. Ion exchange chromatography is commonly used to purify proteins using FPLC.
Size exclusion chromatography
Size exclusion chromatography (SEC) is also known as gel permeation chromatography (GPC) or gel filtration chromatography and separates particles on the basis of size by using porous particles. Smaller molecules enter a porous media and take longer to exit the column, whereas larger particles leave the column faster. It is generally a low resolution chromatography and thus it is often reserved for the final, "polishing" step of a purification. It is also useful for determining the tertiary structure and quaternary structure of purified proteins, especially since it can be carried out under native solution conditions.
Affinity chromatography
Affinity chromatography is based on selective non-covalent interaction between an analyte and specific molecules. Its set to separate all molecules of a specificity from the whole-lot of molecules of a mixture. It is often used in biochemistry in the purification of proteins bound to tags. These fusion proteins are labeled with compounds such as His-tags, biotin, or antigens, which bind to the stationary phase specifically. After purification, some of these tags are usually removed and the pure protein is obtained.
Gas-liquid chromatography
Gas chromatography (GC) is based on a partition equilibrium of analyte between a solid stationary phase and a mobile gas. It involves a vaporised sample being injected onto the chromatography column head. The stationary phase is adhered to the inside of a small-diameter glass tube (a capillary column) or a solid matrix inside a larger metal tube (a packed column). It is widely used in analytical chemistry; though the high temperatures used in GC make it unsuitable for high molecular weight biopolymers, frequently encountered in biochemistry, it is well suited for use in the petrochemical, environmental monitoring, and industrial chemical fields. It is also used extensively in chemistry research.
Countercurrent chromatography
Countercurrent chromatography (CCC) is a type of liquid-liquid chromatography, where both the stationary and liquid phases are liquids. It involves mixing a solution of liquids, allowing them to settle into layers, and then separating the layers. The liquid is passed through coil columns synchronized by coil planet centrifuge. The coiled force keeps the stationary phase against the continuous mobile phase.
Notes
- ↑ Nobel Prize, The Nobel Prize in Chemistry 1952. Retrieved April 7, 2008.
- ↑ W.C. Still, M. Kahn, and A. Mitra. J. Org. Chem. 1978, 43(14): 2923-2925.
ReferencesISBN links support NWE through referral fees
- Jones, Loretta and Peter Atkins. 2000. Chemistry: Molecules, Matter and Change. Gordonsville, VA: W. H. Freeman. ISBN 0716735954
- Kazakevich, Yuri and Rosario LoBrutto, eds. 2007. HPLC for Pharmaceutical Scientists. Hoboken, NJ: John Wiley & Sons. ISBN 0471681628
- Miller, James M. 2005. Chromatography: Concepts and Contrasts. Hoboken, NJ: Wiley-Interscience. ISBN 0471472077
- Poole, Colin F. 2003. The Essence of Chromatography. Amsterdam: Elsevier Science. ISBN 0444501991
External links
All links retrieved December 10, 2023.
- Chromatography Introductory Theory.
- Learning by Simulations Overlapping peaks and their quantification.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.




